Lucence: First Asian firm to clinch US Medicare approval: How Lucence flies the S'pore flag with cancer test kit
First published in The Straits Times on 1 August 2024.
With Enterprise Singapore’s support and network, the precision medicine start-up cracks the competitive US market with innovative blood test invented in the Republic.

In the competitive US healthcare industry dominated by multibillion-dollar companies, a Singapore precision medicine start-up is making its mark.
In 2023, Lucence made the news by becoming the first Asian-headquartered company to have its cancer test approved by Medicare, the US government-funded healthcare insurance programme.
It not only made Lucence’s solution more accessible to everyday Americans, but also paved the way for its tests to be approved by other insurance providers in the market.
Lucence founder and chief executive officer Tan Min-Han, 49, says: “The annual expenditure of Medicare is about US$848 billion (S$1.14 trillion). The fact that the Lucence test is regarded as useful to US patients and that the US government will cover it… this is the most important step in any healthcare invention.”
Invented in Singapore, Lucence’s LiquidHallmark is a blood test that profiles 80 genes and 15 types of cancers. The liquid biopsy test helps match late-stage cancer patients with targeted drugs – should there be any – while being less invasive than conventional tissue testing.
The tests are also available at most private and public hospitals and clinics in Singapore, as well as at medical facilities in Hong Kong, Taiwan and Canada. Lucence’s Singapore lab serves as a base, receiving and processing blood samples from these various clinics across Asia and Canada.
Connecting to key US partners
Today, the start-up is looking to strengthen its hold in the US, a front runner in the US$75 billion global precision medicine market, where treatment is tailored according to one’s genetic make-up and lifestyle.
To navigate the vast and complex US market, Lucence is strategically seeking partners for commercialisation. Dr Tan acknowledges intricacies posed by the country’s diverse market of 50 states, each presenting unique opportunities and challenges.
He says: “Partnerships are always the right way in the US, because to do it alone in a country that's geographically dispersed, can be challenging.”
The firm is also tapping Enterprise Singapore’s (EnterpriseSG) network in the US to scale more quickly, with the latter’s overseas offices (see “Supporting firms global ambitions”) helping Lucence to establish strong working relationships with US institutions.

This has been invaluable as Lucence expands its reach.
“We are at this point where we are reaching out to many of the different hospitals.” says Dr Tan. “I think EnterpriseSG is definitely a valued partner as a central hub of sorts, the go-to contact point for Singapore companies.”
Supporting firms’ global ambitions
Enterprise Singapore opened its new San Francisco Overseas Centre in November 2023 to help companies like Lucence establish a presence in the Bay Area. It is the agency’s third US office, alongside those in New York and Los Angeles.
It provides companies preparing for overseas expansion with market insights and guidance on local regulations and business practices. Once abroad, Overseas Centres connect businesses to local partners and facilitate networking opportunities.
To date, there are close to 200 Singapore companies with a business presence in the US. They come from a wide range of sectors including healthcare, fintech, consumer tech manufacturing, food services and retail.
From blood test to saliva test: Pivoting to stay afloat
Lucence clocked over US$6 million in revenue in 2023, doubling its earnings from 2022. Starting with five staff members in 2017, it has grown to become a 73-strong team.
Now operating from dual headquarters in Singapore and Palo Alto, in California, Lucence’s success comes after a rocky journey into the US market in 2020.
In 2020, it had started building a second laboratory in Palo Alto to complement its Singapore flagship. But supply chain issues brought on by the Covid-19 pandemic slowed the arrival of equipment and materials, and priority was also given to labs setting up Covid-19-testing facilities.
“It was just very painful,” Dr Tan recalls. “Anything that would have taken one week would be delivered in six weeks to eight weeks, or would never come.”
Despite these setbacks, he found a way to keep the company afloat while sticking to his mission to save lives. The company pivoted to developing the first saliva-based Covid-19 test for Singapore as an alternative to solve problems with painful, invasive and inconvenient nasal swabbing.
EnterpriseSG also provided Lucence with an internationalisation grant, which helped in the set-up of its Palo Alto laboratory in 2020.
Dr Tan says: “The funding support for companies internationalising has always been a challenge because the US is an expensive environment.”
When the pandemic finally stabilised, the Lucence team charged ahead to continue to grow LiquidHallmark. It also developed a second product, LucenceInsight, which can detect 50 types of cancers with a single blood test.
Making cancer screening less invasive
At the heart of Lucence is Dr Tan’s vision of helping people fight cancer.
Dr Tan has worked in oncology since 1999. He was a consultant medical oncologist at the National Cancer Centre Singapore when he was struck by the number of late-stage cancer patients coming through the clinic door. It was too late to help many of them as the cancer had spread too deeply.
“They were coming to my office breathless, or they had not seen any other doctor,” says Dr Tan. “I felt that I could have done more if they had come to me earlier.
“In early-stage cancer, you have an 80 per cent chance of survival in the long term. In late-stage cancer, it’s maybe 20 per cent.”

However, he saw that people avoided early cancer screening as they found tests such as mammograms or colonoscopies inconvenient or invasive.
Furthermore, many cancers are not covered by traditionally recommended screenings during regular health check-ups.
“Around eight out of 10 deaths caused by cancer today are not screened for with conventional screening approaches,” he says. “That can include liver, pancreas and nasopharyngeal cancer. The effective recommendation today is to see your doctor when you have symptoms.”
Dr Tan chose to do more research to address the issue. He says: “I find it fulfilling to treat patients. But I also recognised that the deep issues of healthcare, such as early detection, needed to be solved on a much more fundamental and technological level.”
While he practised medicine as an oncologist, he dove into medical research, too, and was awarded a PhD from NUS. He has since published about 100 research papers on breast, lung, gastrointestinal, liver, prostate, kidney, and head and neck cancers.
In 2011, he was appointed to lead a research team at the Agency for Science, Technology and Research (A*Star), and in 2017, Lucence was spun out of the team.
That was the same year that his brother Tan Min-Liang led gaming firm Razer to its initial public offering in Hong Kong.
This proximity to a successful tech enterprise proved valuable for Dr Tan’s own venture. “I had the great fortune of having a ringside view of how Razer grew, and I got a sense of the efforts required,” he says. “You need to work closely with folks who have a lot of experience commercialising products.”
However, he views Lucence’s journey as more than just the company’s individual success. It is part of Singapore’s growing precision medicine ecosystem.
“We’re very glad to be working with EnterpriseSG to conceptualise what this vision looks like in the region and farther afield in the US,” he says, adding that a strong ecosystem requires academia, government and companies to work together.
“EnterpriseSG plays a crucial role in helping Singapore build its world champions. Through our partnership, we hope to see more diagnostic and pharmaceutical companies join the ecosystem.”
Dr Tan acknowledges that with an ageing population globally, the demand for tailored medical solutions is increasing. “There’s a growing need for precision medicine, and we’re laying a strong foundation for this.”
The business of precision medicine: Balancing commercialisation with innovation
One of the commercial challenges for Lucence is to bring down the cost and speed of genetic testing for cancer.
Dr Tan says: “While our tests for patients cost anywhere from $2,000 to over $5,000, and it takes one to two weeks, this is an improvement over the speed and cost of a tissue biopsy, which could take four to five weeks and cost over $10,000.
“Over the years, we’ve worked with many good business folks to reduce our costs. Scaling up means making the tests more affordable.”

Being business-savvy about commercialising its products has helped Lucence to fend off the steep competition. Dr Tan says there are over 200 companies developing liquid biopsy products at the time of writing.
To win Medicare approval, Lucence also leveraged the Asian markets to leap ahead in testing and validation of its technologies.
“We started deploying our technology to help patients in Hong Kong and Singapore much earlier,” notes Dr Tan, adding that cancer patients and doctors in the region demand the best technology.
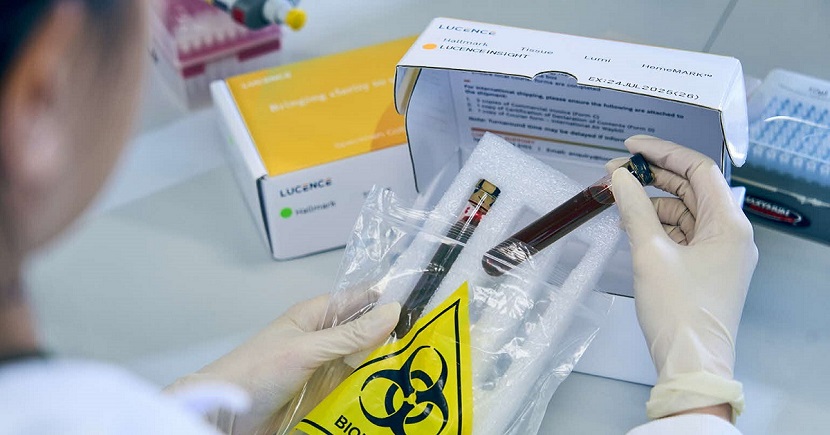
Many saw the benefits of Lucence’s liquid biopsy test and opted for it. The firm then continued to improve its product through multiple iterations and experience.
Results from head-to-head studies against other US-approved cancer tests provided strong data to support LiquidHallmark for insurers and oncologists.
“A business's purpose is to solve people's needs,” says Dr Tan. “We will solve the needs of many more people worldwide for earlier cancer detection. We are only at 1 per cent of our journey.”
This is part of a series showcasing how Enterprise Singapore empowers home-grown businesses in their defining moments of growth, helping them transform, innovate and go global. Find out more here.

